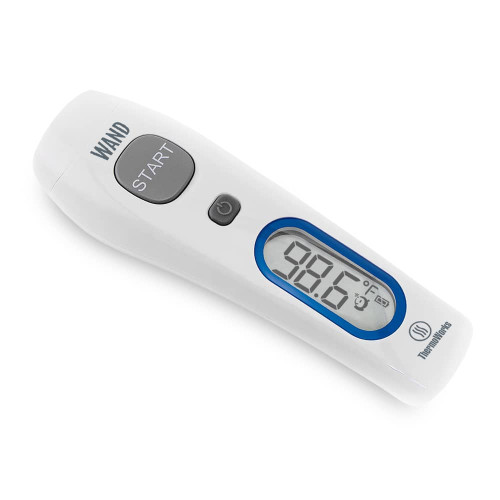
Infrared Technology: No-touch temperature readings
Safely get temperature readings with zero contact necessary. Simple and sanitary.
High Accuracy: Blows the competition away
WAND is accurate to ±0.4°F across the range of human temperatures.
FDA-Cleared*: Monitor your health with confidence
WAND is a Professional Class II medical device cleared for safe use on both adults and children.
Easy to Use: Turn it on and press a button
Takes temperature readings with the touch of a button. No setup required. Big bold backlit display.
Soft “Ready” Beep: Confirms your reading
Take the temperature of your sleeping child without disturbance, or mute the “ready” beep all together.
*ThermoWorks Wand Thermometer measures infrared energy radiated from the skin at the center of the forehead area. This captured energy is collected through the lens and converted to a body temperature value (displays oral equivalent temperature).
Thanks to large quantity savings and reduced transportation costs, we are passing along a cost reduction on the ThermoWorks WAND Forehead Thermometer. Already one of our biggest sellers, we are now producing volumes that give us some significant savings. While we believe that WAND was a great professional value at the previous price, going forward we are excited to save our customers some money!
- One-second readings
- WAND digital forehead thermometer is suitable for temperature screening in homes, hospitals, businesses, warehouses, schools, and physical examination centers.
- Gives accurate, consistent oral-equivalent results for all ages
- Surface mode for surface temps of other common items
- High temperature alert and mute setting
- Bright LED backlight
- Stores 25 readings
WAND non-contact forehead thermometer gives you proven accuracy when you need it most. Simple to use with readings in 1 second means you get the important information you need quickly.
* ThermoWorks WAND holds an FDA device registration number and has the designation FDA-Cleared. WAND is categorized as a Class II medical device under the category listing 'Thermometer, Electronic, Clinical'. The FDA-Approval designation is only given to Class III products like drugs, pacemakers, and vaccines that pose a higher risk to humans, and require a different level of testing and approval. Class I and II products are not tested to the same level and are “cleared”.
ThermoWorks WAND gives you proven accuracy when you need it most. Take adult and child forehead temps quickly with no-touch infrared technology for safety and hygiene. No need to disturb a sleeping child. Simply hold the unit about an inch away from the center of the forehead, press Start and in 1 second you'll have an accurate reading. No waiting.
The blue backlight makes temperatures easy to read, even in the dark. WAND stores your last 25 readings for easy recall. Automatically reads “oral equivalent” temperatures. Change to surface mode and measure surface temps of other common items to 176°F (80°C).
ThermoWorks has already helped tens of thousands of school districts, universities, restaurants, offices, plants, non-profits, clinics and government agencies across the nation to screen staff and patrons for a safe return to business. With so much riding on keeping your operations safe, don't mess around with second best. Put your trust in ThermoWorks.
| Specifications |
|---|
| Forehead Range | 93.2 to 108°F (34 to 42.2°C) |
|
| Surface Range | -7.6 to 176°F (-22 to 80°C) |
| Operating Range** | 50 to 104°F (10 to 40°C) 15 to 85% RH |
| Storage Range | -4 to 122°F (-20 to 50°C) RH≤85% |
|
| Resolution | 0.1° |
| Forehead Accuracy* | ±0.4°F (±0.2°C) within 95 to 107.6°F (35 to 42°C); otherwise ±0.5°F (±0.3°C) |
| Surface Accuracy* | ±0.5°F (±0.3°C) within 71.6 to 108°F (22 to 42.2°C); otherwise ±4% or ±4°F (±2°C), whichever is greater |
| Emissivity | Fixed at 0.95 in Surface Mode |
| Spectral Range | 5.5 µm |
| Temperature Units | °C and °F switchable |
| Response Time | 1 second |
| Approvals | FDA, Class II – Clinical, Cleared (USA)
Health Canada, Class II Medical Device (Canada) |
| Auto-Off | 60 seconds |
| Battery | 2x AAA - included, 3000 hours |
| Dimensions | 6.2 H x 1.9 W x 1.6 D (158 H x 48 W x 40.2 D mm ) |
| Weight | 3.5 oz. (100 g) |
**EMC/RFI:Readings may be affected if the unit is operated within radio frequency electromagnetic field strength of approximately 3 volts per meter, but the performance of the instrument will not be permanently affected.
*FDA-cleared for safety & accuracy for kids and adults. ThermoWorks Wand Thermometer measures infrared energy radiated from the skin at the center of the forehead area.This captured energy is collected through the lens and converted to a body temperature value (Displays oral equivalent temperature).
It’s helpful to know each individual’s normal temperature when they are well. This is the best way to accurately diagnose a fever. To get a reference value, we suggest takingmultiple readings (5 to 10) over one minute, taking the highest number to determine “normal temperature”.
Downloads
 Download WAND Operating Instructions
Download WAND Operating Instructions
 Download WAND Info Sheet
Download WAND Info Sheet
 Tips for using WAND Forehead Thermometer
Tips for using WAND Forehead Thermometer
 Download WAND Quick Reference
Download WAND Quick Reference
FDA Device Listing Number: D383100
Pre-market Submission, 510(k): K121428
Classification: Class II, Thermometer Electronic Clinical
Is WAND ‘FDA-Approved’?
The FDA does not “Approve” moderate-risk medical devices (Class II) like dialysis equipment, many types of catheters, and forehead thermometers. Instead the FDA “Clears” Class II devices once they have demonstrated that the device is substantially equivalent to a legally marketed device. Class III devices that are in the higher-risk category such as mechanical heart valves, and implantable infusion pumps generally require FDA approval. The FDA has provided the article, Is It Really ‘FDA-Approved’ for more information.
From section 502 of the Federal Food, Drug and Cosmetic Act (FFDCA) which covers device misbranding: There is any representation that creates an impression of official approval because of the possession by the firm of an FDA registration number.
WAND holds an FDA device registration number and is ‘FDA-Cleared’ under the Class II designation ‘Thermometer, Electronic, Clinical’.
Any Class II device claiming that it is ‘FDA-Approved’ clearly violates the FDA’s misbranding guidelines.
Why doesn’t ThermoWorks use the FDA Logo like I’ve seen used by other forehead thermometer suppliers?
“Because it may violate federal law and could subject those responsible to civil and/or criminal liability.” Here is the introductory paragraph to FDA’s Logo Policy:
The FDA logo is for the official use of the U.S. Food and Drug Administration (FDA) and not for use on private sector materials. To the public, such use would send a message that FDA favors or endorses a private sector organization or the organization’s activities, products, services, and/or personnel (either overtly or tacitly), which FDA does not and cannot do. Unauthorized use of the FDA logo may violate federal law and subject those responsible to civil and/or criminal liability.
Use of the FDA logo is strictly prohibited, and those who do are in clear violation of FDA’s Logo Policy.